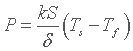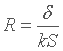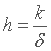www.leguman.ht.st
| head losses |
pumps |
convection |
| Conduction + Convection |
| Wb effect on the fluid flow |
| Radiation |
©
2001 - 2002.
|
| previous | ||
|
The truth about h ... In order to finally be able to give an interpretation to this coefficient,
and for ease of understanding, we will assume that the two boundary
layers coincide. We also will assume that the fluid is slowed down
so much there that we can consider that the heat exchange is done
by pure conduction. Under these circumstances the fluid will behave
from the thermal point of view like a solid and the heat transfer
will obey the Fourier's Law.
Here S is still the contact surface area between solid and fluid and k is the thermal conductivity of the fluid. Thus thermal resistance can be written :
If we remember the expression of the "true" thermal resistance for convection, seen here, we can finally write :
Lastly, something to chew on in connection with h
! We were looking for high values of h.
We can thus interpret (and that's all we can do since this relation
is just a trick to understand qualitatively) that the lower d
is and the higher k
is, the more h
is increased. Just a little comment, rigorously it would be necessary to talk about thermal conducto-convection, because, as we just saw before, heat exchange between a solid and a fluid simultaneously involve the phenomenon of conduction and convection, both being closely coupled. But the convective transfer coefficient also depends on viscosity,
on density, and on the specific heat of the fluid. Unfortunately
to analyze how these properties will influence h
it would be necessary to return to a more theoretical analysis,
this is not the goal of these articles. |
||
| previous |