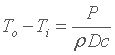www.leguman.ht.st
| head losses |
pumps |
convection |
| Conduction + Convection |
| Wb effect on the fluid flow |
| Radiation |
©
2001 - 2002.
|
| previous | ||
|
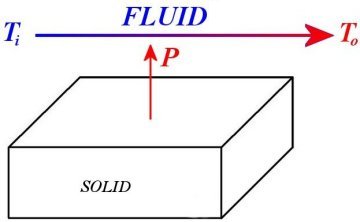
First principle of thermodynamics Considering its most simplified version, it establishes the relationship between :
The thermal power P is proportional to all the remainder, and that gives :
P
and Ti
are given and do not depend on the cooler considered
(P
depends on the CPU and Ti
from what occurs before the input). We are looking
for the coldest solid surface, therefore the fluid temperature should
also be the coldest possible along its course.
then a better flow rate D, a better density r and a better specific heat c is desired (actually the product of both). Concerning flow rate, this joins what we saw thanks to the interpretation with the Fourier's Law, i.e. the more it is, the more velocity also is. With regard to the two remainders, density and specific heat, it depends entirely on the choice of the fluid, since they are physical properties. The last thing in connection with this principle, let us compute To - Ti and you will notice that this difference is rather small, generally smaller than 1 °C for water with the usual flow rate of a water cooling system. |
||
| previous |